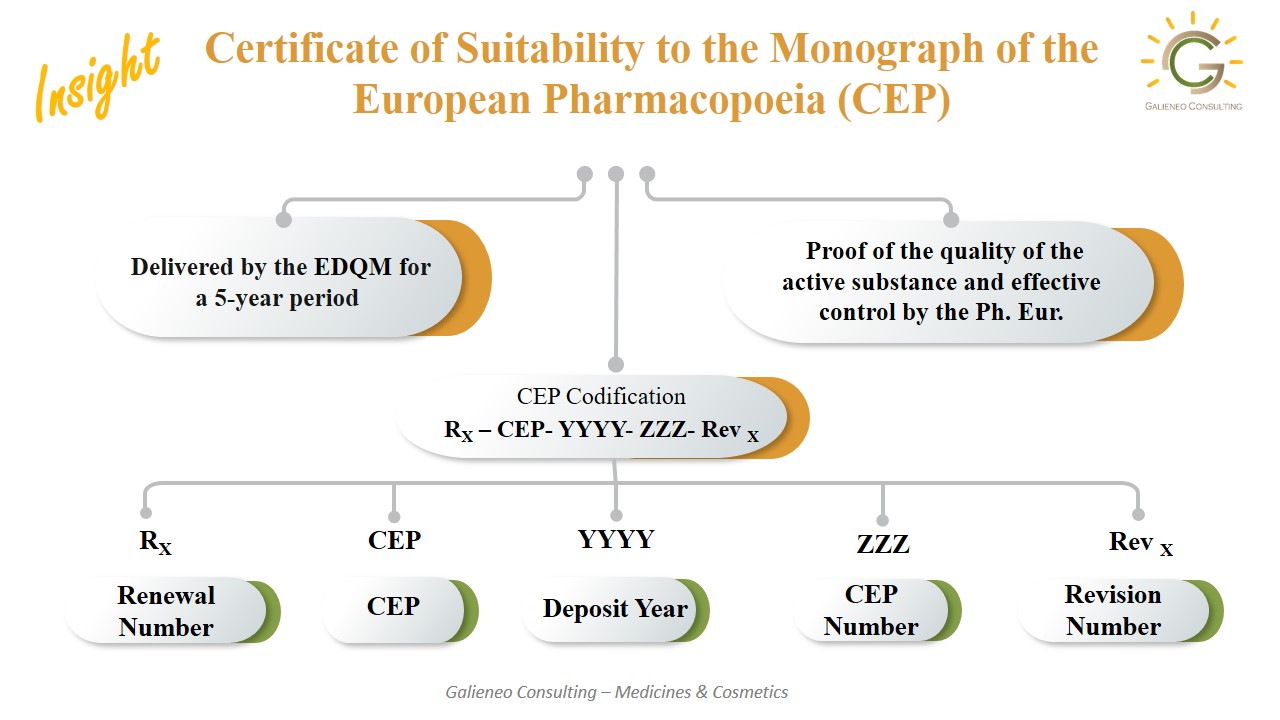
The Certificate of Suitability to the European Pharmacopoeia (CEP) proves that the quality of a pharmaceutical active substance or excipient complies with the quality described in the applicable monograph of the European Pharmacopoeia. Manufacturers of pharmaceutical products submit the CEP to the competent authorities during the authorization procedure, as part of the information on the active substance or excipients. The holder of the CEP, the manufacturer of the active substance or excipient, is responsible for its application and the complete lifecycle of the document.
A CEP is not a Good Manufacturing Practices (GMP) certificate and does not substitute for a GMP certificate. Nevertheless, the CEP applicant must ensure that the active substance or excipient has been manufactured in compliance with GMP.